At the core of our innovation lies a multiplex approach:
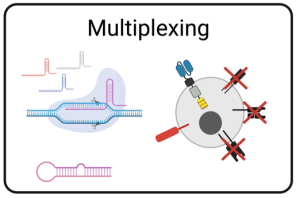 The utilization of singleplex cell engineering serves as the foundation for most cell therapy products in both the development and commercial phases. However, a singular modification to cells may not provide adequate persistence or effectively address the complexities of diseases such as solid tumors with metastatic characteristics. By optimizing multiplex cell and gene engineering methods, we fortify immune cells with augmented capabilities, enhancing persistence, efficacy, and safety.
The utilization of singleplex cell engineering serves as the foundation for most cell therapy products in both the development and commercial phases. However, a singular modification to cells may not provide adequate persistence or effectively address the complexities of diseases such as solid tumors with metastatic characteristics. By optimizing multiplex cell and gene engineering methods, we fortify immune cells with augmented capabilities, enhancing persistence, efficacy, and safety.
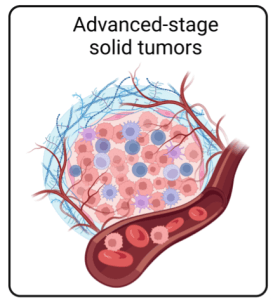 We prioritize addressing the unmet medical needs in oncology, with a focus on solid tumors. The complex and diverse nature of the solid tumor microenvironment presents a formidable challenge for cellular therapies. Our lab utilizes advanced cell and gene engineering techniques to create next-generation CAR T cells capable of withstanding and overcoming the tumor microenvironment. Our research also encompasses the development of cutting-edge gene delivery methods to enhance the efficacy, safety, and cost-effectiveness of cellular immunotherapies.
We prioritize addressing the unmet medical needs in oncology, with a focus on solid tumors. The complex and diverse nature of the solid tumor microenvironment presents a formidable challenge for cellular therapies. Our lab utilizes advanced cell and gene engineering techniques to create next-generation CAR T cells capable of withstanding and overcoming the tumor microenvironment. Our research also encompasses the development of cutting-edge gene delivery methods to enhance the efficacy, safety, and cost-effectiveness of cellular immunotherapies.
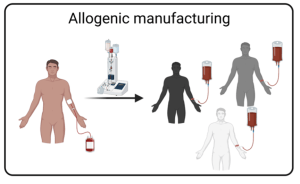 The current complex manufacturing process of autologous cell therapies and their patient-specific nature limit their scalability and accessibility. Our lab focuses on developing allogeneic CAR T cell products as a solution, using healthy donor material for multiple patients to increase accessibility and availability. We use multiplex gene engineering to enhance the functional and targeting capabilities of these cells and introduce modifications to avoid recognition and elimination by the host immune system.
The current complex manufacturing process of autologous cell therapies and their patient-specific nature limit their scalability and accessibility. Our lab focuses on developing allogeneic CAR T cell products as a solution, using healthy donor material for multiple patients to increase accessibility and availability. We use multiplex gene engineering to enhance the functional and targeting capabilities of these cells and introduce modifications to avoid recognition and elimination by the host immune system.
Clinical Translation
We are seamlessly integrated into the USC clinical and translational environment, with the advantage of access to the state-of-the-art USC/CHLA Good Manufacturing Practice (GMP) facility. As GMP manufacturing is a critical challenge in the advancement of cell therapy treatments, we leverage our extensive research, product development, and GMP production knowledge in the earliest stages of design to expedite product translation and bring these innovative treatments to the clinic with speed and efficiency.
Visit the USC/CHLA Cell and Gene Therapy Program webpage to find out more about clinical translation at USC.
Evidence Synthesis & Regulatory Science
Our lab places a significant emphasis on incorporating evidence synthesis methodologies, including computational biology, cost-effectiveness analysis, mathematical modeling, systematic reviews, and meta-analyses, in the assessment of the safety and efficacy of cell-based therapies. These techniques afford a thorough examination of both preclinical and clinical data and aid in identifying potential threats to reproducibility. Furthermore, the utilization of these techniques can contribute to improving regulatory frameworks and the creation of predictive models, which can support the decision-making process and streamlining cell therapy development.
Our lab is equally committed to examining the ethical and policy-related issues associated with cell-based therapies. With the recent surge of unethical practices by dubious clinics offering unproven stem cell products to vulnerable patients, our work in policy is dedicated to addressing this pressing concern. We aim to identify the gaps in current regulations and provide effective solutions to eliminate this dangerous phenomenon, which undermines the value and credibility of stem cell therapies that undergo rigorous testing. We are also dedicated to advancing regulatory science and our efforts are aimed at harmonizing and strengthening existing regulations to promote the rapid and safe deployment of cell therapies.
